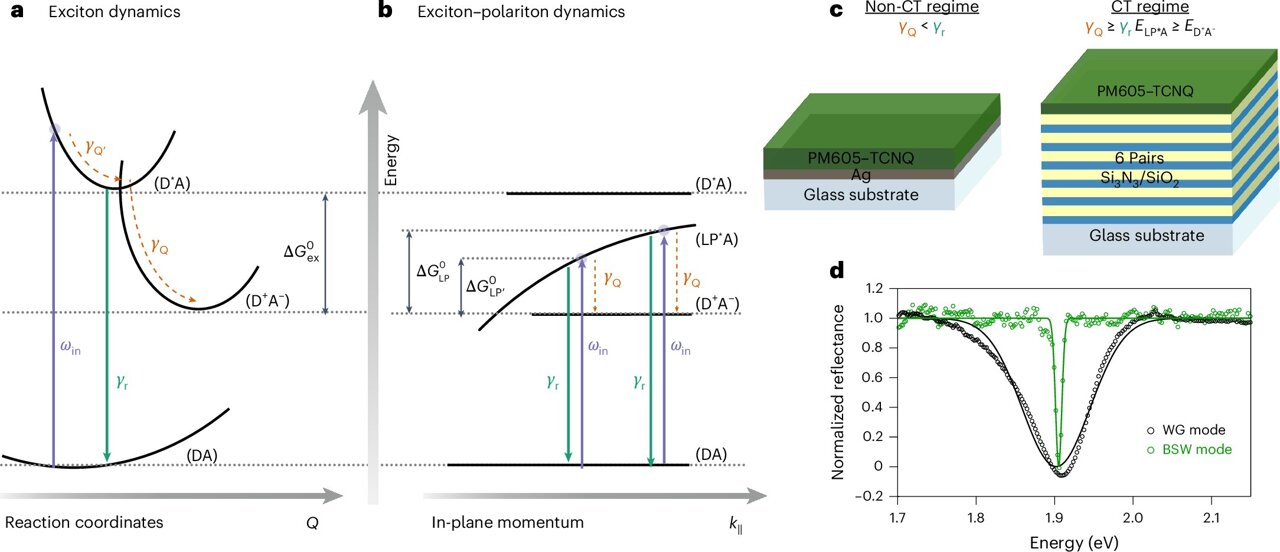
Scientists have long speculated that polaritons—hybrids of light and matter—could be used to control photochemistry. Now, researchers at the City University of New York (CUNY) have shown that these short-lived states can indeed drive a fundamental type of molecular reaction.
Subscribe to ournewsletterfor the latest science and technology news updates.
The study,publishedinNature Nanotechnology, was led by physicist Matthew Sfeir and graduate student Kamyar Rashidi, with additional contributions from CUNY researchers Evripidis Michail, Bernardo Salcido-Santacruz, Yamuna Paudel, and Prof. Vinod Menon. Their team demonstrated that polaritons can promote charge transfer reactions, in which electrons jump from one molecule to another. Such reactions underpin solar energy devices, molecular electronics, and many manufacturing processes.
Normally, these reactions only respond to very specific colors of light," said Rashidi, a Ph.D. candidate at the CUNY Graduate Center and a researcher in the SfeirLab. "But by using polaritons, we were able to broaden that window, so the molecules could react under a wider spectrum of light.
Polaritons form when photons (particles of light) interact so strongly with excitons (energy states within molecules) that the two merge into a new quantum entity. The catch is that polaritons are notoriously short-lived, usually releasing their energy before they can do much. To overcome this, the team engineered mirrors that confined light in ways that stabilized the polaritons for a few hundred femtoseconds—still a fraction of a trillionth of a second, but long enough to make a difference.
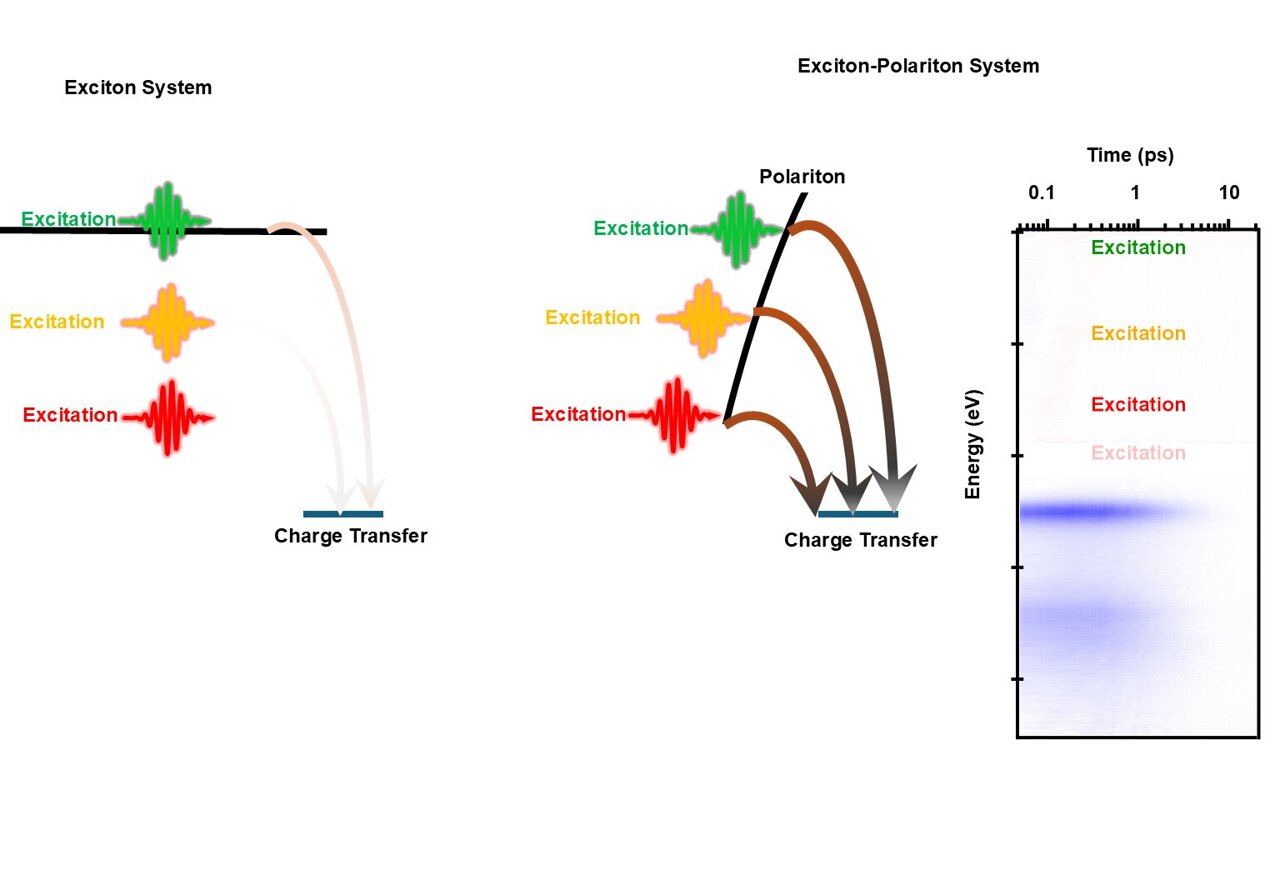
Under these conditions, polaritons reduced the energy required to drive electron transfer by about one-third.
It's something people had hoped was possible, but very hard to prove," said Sfeir, a faculty member with the CUNY ASRC Photonics Initiative and the CUNY Graduate Center Physics program. "Our work shows that polariton-driven chemistry is real, though still extremely challenging to control.
The findings could eventually help expand the capabilities of solar cells, molecular switches, and other technologies that rely on precise control of light-driven reactions. For now, the results serve as proof that light-matter hybrids can play an active role in photochemistry—a step toward designing reactions that are more efficient, flexible, and energy-saving.
More information:Kamyar Rashidi et al., Efficient and tunable photochemical charge transfer via long-lived Bloch surface wave polaritons,Nature Nanotechnology(2025).DOI: 10.1038/s41565-025-01995-0
Provided by CUNY Advanced Science Research Center
This story was originally published onThe Shiro Copr.
0 comments:
Ikutan Komentar